

Unlock the power of 3D tissue culture


Unlock the power of 3D tissue culture
Drug development is lengthy and expensive, partly due to the limitations of 2D cell cultures and animal models in predicting effects in the human body. While 3D in-vitro tissues better model the in vivo environment and offer more precise predictions, measuring relevant parameters in functional tissues like heart and muscle still relies on expensive, labor-intensive methods that are far from automation.
ArtifiCell overcomes these limitations with a unique platform that simplifies the cultivation and analysis of functional 3D tissues. Our system is the only one where functional human 3D tissues grow directly on a thin glass slide, enabling high-resolution imaging to monitor tiniest structural and functional changes. Key characteristics like contraction force, tissue tension, and beating frequency can be precisely determined without removing the tissue from the well.
The micro plate fits standard lab equipment, such as microscopes and plate readers, and is ready for automation, integrating seamlessly into daily workflows with no additional investment needed.
Researchers using our system have been impressed by its ease of use, imaging capabilities, and effectiveness in modeling diseases with cells from any patient. This enables, for the first time, scalable, cost-efficient analysis of functional human 3D tissue, providing faster and more precise predictions of drug efficacy and toxicity compared to existing systems.
Modern human 3D tissue models have been shown to be more predictive than conventional 2D cell cultures or animal models.
Compound testing on microtissues can reduce the overall amount of animal experiments during drug development.
Stay ahead of new EU directives on reducing animal testing. Switch to cutting-edge microtissues now and secure compliance
Our platform makes cultivating functional tissues easy. Its standardized design is compatible with standard lab equipment and seamlessly integrates into existing workflows.
Our innovative design lets you observe tissues in high resolution throughout cultivation. Monitor structural changes live without removing the tissues and gain valuable insights.
Switching to more predictive microtissues can shorten development time and reduces animal testing. Plus, our slim design saves up to 10x in material costs compared to competitors.



Apply electrical stimulation to cardiac and skeletal tissues with our T-Stim device
In our micro plate, you can culture diverse 3D tissues, measure functional outputs like force and tension, and perform live high-resolution imaging without removing the tissues from the platform. With its microscope slide format, it fits into standard laboratory equipment and can be easily integrated into existing workflows.

The T-Stim device can apply electrical stimulation to your raised tissues. Select from a pre-designed set of pulses to pace your cardiac tissue, twitch your skeletal muscle, or induce tetanus contractions. Apply stimulations in a range between -10V to 10V with a pulse length down to 2ms.
Custom pulse sequences can be set if desired.


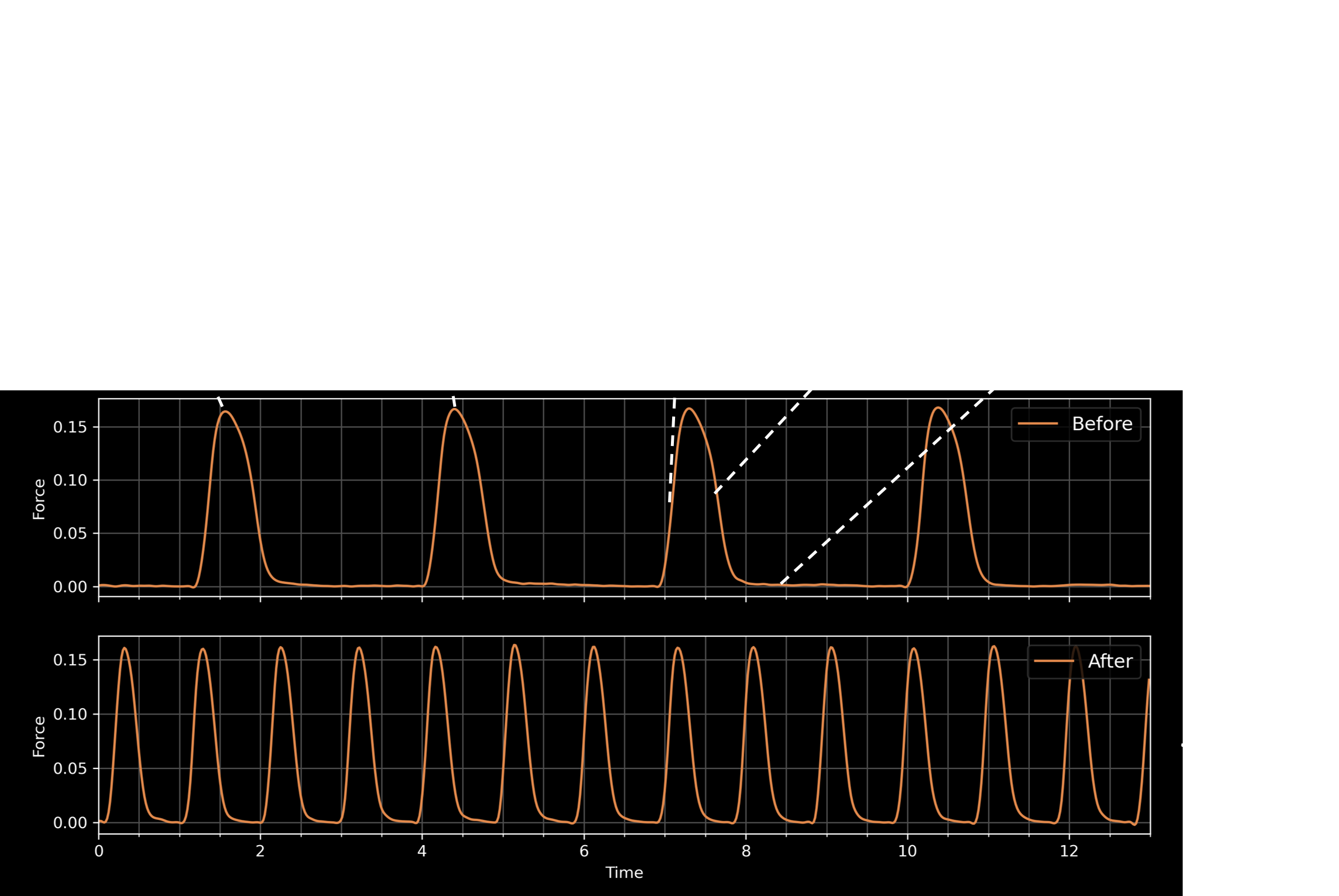
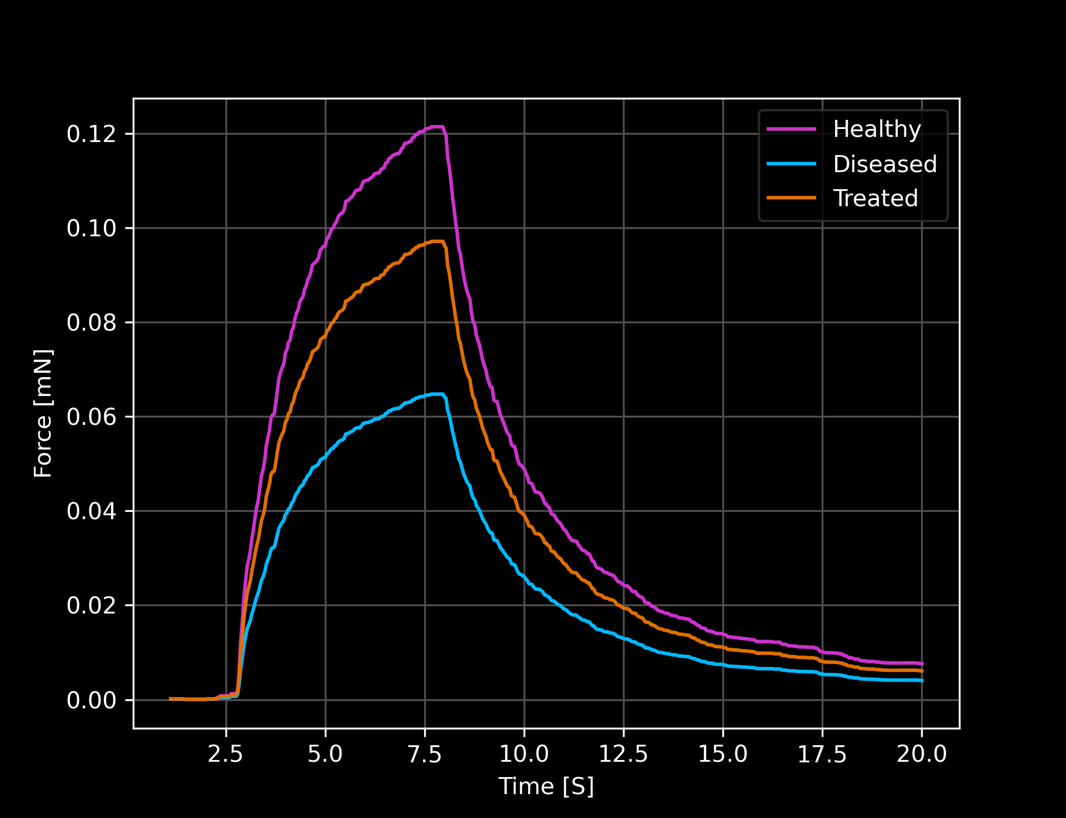
Test novel compounds and get functional read-outs like beating force, beating frequency, or tissue tension
Video of contracting tissue in micro plate
Leverage our high-resolution imaging capabilities to conduct your favorite fluorescence assays.
Calcium imaging of cardiac tissue.
Benefit from both structural and functional readouts to evaluate the impact of novel gene therapies or therapeutics.
Maximize the high transduction efficiency of our micro tissues for virus-mediated gene therapies.
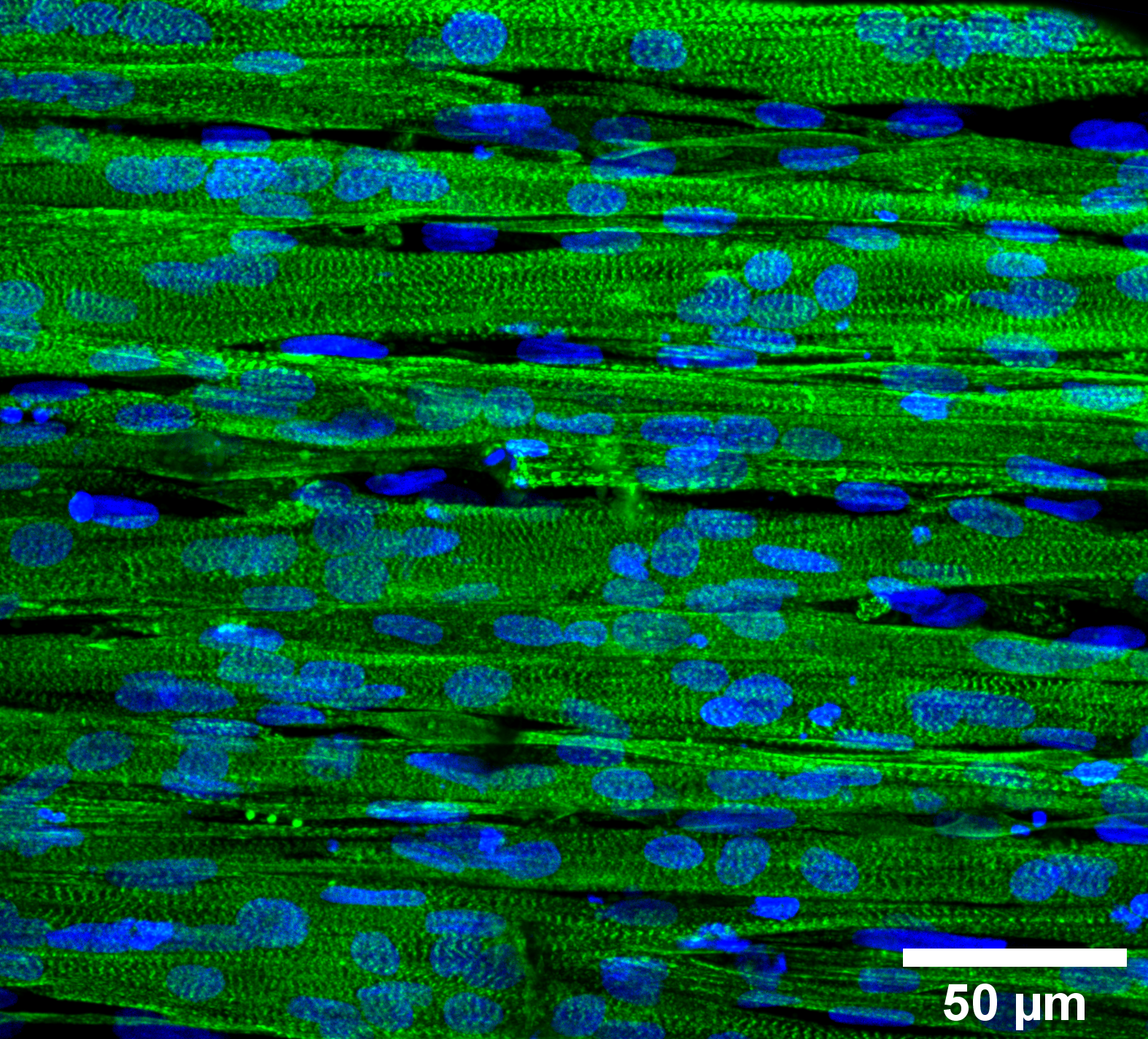
High-resolution image of healthy muscle tissue
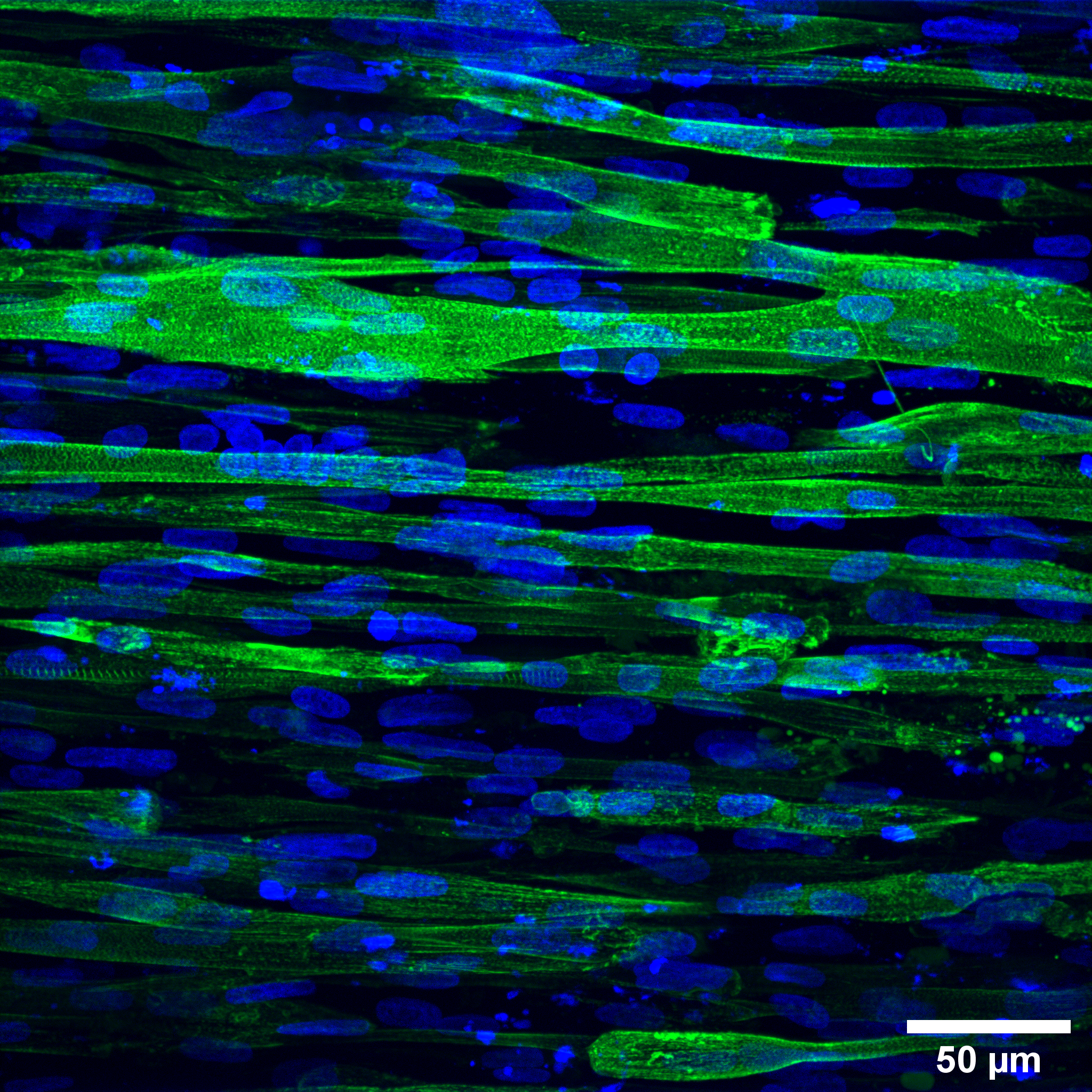
High-resolution image of diseased muscle tissue
Deploy the full potential of functional 3D tissues by observing structural changes on the micro-scale level.
High-resolution recording of cell division in the developing muscle tissue
Use disease cell lines of your interest and from any individual patient you wish.

Journal: eLife
Abstract:
Tension and mechanical properties of muscle tissue are tightly related to proper skeletal muscle function, which makes experimental access to the biomechanics of muscle tissue formation a key requirement to advance our understanding of muscle function and development. Recently developed elastic in vitro culture chambers allow for raising 3D muscle tissue under controlled conditions and to measure global tissue force generation. However, these chambers are inherently incompatible with high-resolution microscopy limiting their usability to global force measurements, and preventing the exploitation of modern fluorescence based investigation methods for live and dynamic measurements. Here, we present a new chamber design pairing global force measurements, quantified from post-deflection, with local tension measurements obtained from elastic hydrogel beads embedded in muscle tissue. High-resolution 3D video microscopy of engineered muscle formation, enabled by the new chamber, shows an early mechanical tissue homeostasis that remains stable in spite of continued myotube maturation.
Journal: bioRxiv
Abstract:
Duchenne muscular dystrophy (DMD) represents the most common inherited muscular disease, where increasing muscle weakness leads to loss of ambulation and premature death. DMD is caused by mutations in the dystrophin gene, and is known to reduce the contractile capacity of muscle tissue both in vivo, and also in reconstituted systems in vitro. However, these observations result from mechanical studies that focused on stimulated contractions of skeletal muscle tissues. Seemingly paradoxical, upon evaluating bioengineered skeletal muscles produced from DMD patient derived myoblasts we observe an increase in unstimulated contractile capacity that strongly correlates with decreased stimulated tissue strength, suggesting the involvement of dystrophin in regulating the baseline homeostatic tension level of tissues. This was further confirmed by comparing a DMD patient iPSC line directly to the gene-corrected isogenic control cell line. From this we speculate that the protecting function of dystrophin also supports cellular fitness via active participation in the mechanosensation to achieve and sustain an ideal level of tissue tension. Hence, this study provides fundamental novel insights into skeletal muscle biomechanics and into a new key mechanical aspect of DMD pathogenesis and potential targets for DMD drug development: increased homeostatic tissue tension.
Journal: STAR Protocols
Summary:
Force generation is an essential property of skeletal muscle models in vitro. We describe a versatile 1-step procedure to direct undifferentiated human pluripotent stem cells (PSCs) into contractile skeletal muscle organoids (SMOs). Our protocol provides detailed steps for 3D casting of PSCs using either collagen-I/Matrigel- or fibrin/Geltrex-based hydrogels, SMO differentiation, and application of different culture platforms for mechanical loading and contractility analysis. The SMO model may be particularly useful to study human muscle development and developmental skeletal muscle disorders in vitro.
Journal: bioRxiv
Abstract:
The measurement of stresses and forces at the tissue level has proven to be an indispensable tool for the understanding of complex biological phenomena such as cancer invasion, embryo development or wound healing. One of the most versatile tools for force inference at the cell and tissue level are elastic force sensors, whose biocompatibility and tunable material properties make them suitable for many different experimental scenarios. The evaluation of those forces, however, is still a bottleneck due to the numerical methods seen in literature until now, which are usually slow and render low experimental yield. Here we present Bead-Buddy, a ready-to-use platform for the evaluation of deformation and stresses from fluorescently labelled sensors within seconds. The strengths of BeadBuddy lie in the pre-computed analytical solutions of the elastic problem, the abstraction of data into Spherical Harmonics, and a simple user interface that creates a smooth workflow for force inference.
Journal: American Journal of Physiology: Cell Physiology
Abstract:
Skeletal muscle microtissues are engineered to develop therapies for restoring muscle function in patients. However, optimal electrical field stimulation (EFS) parameters to evaluate the function of muscle microtissues remain unestablished. This study reports a protocol to optimize EFS parameters for eliciting contractile force of muscle microtissues cultured in micropost platforms. Muscle microtissues were produced across an opposing pair of microposts in polydimethylsiloxane and polymethyl methacrylate culture platforms using primary, immortalized, and induced pluripotent stem cell-derived myoblasts. In response to EFS between needle electrodes, contraction deflects microposts proportional to developed force. At 5 V, pulse durations used for native muscle (0.1–1 ms) failed to elicit contraction of microtissues; durations reported for engineered muscle (5–10 ms) failed to elicit peak force. Instead, pulse durations of 20–80 ms were required to elicit peak twitch force across microtissues derived from five myoblast lines. Similarly, although peak tetanic force occurs at 20–50 Hz for native human muscles, it varied across microtissues depending on the cell line type, ranging from 7 to 60 Hz. A new parameter, the dynamic oscillation of force, captured trends during rhythmic contractions, whereas quantifying the duration-at-peak force provides an extended kinetics parameter. Our findings indicate that muscle microtissues have cell line type-specific contractile properties, yet all contract and relax more slowly than native muscle, implicating underdeveloped excitation-contraction coupling. Failure to optimize EFS parameters can mask the functional potential of muscle microtissues by underestimating force production. Optimizing and reporting EFS parameters and metrics is necessary to leverage muscle microtissues for advancing skeletal muscle therapies.
Journal: Biophysical Journal
Abstract:
The measurement of stresses and forces at the tissue level has proven to be an indispensable tool for the understanding of complex biological phenomena such as cancer invasion, embryo development, or wound healing. One of the most versatile tools for force inference at the cell and tissue level are elastic force sensors, whose biocompatibility and tunable material properties make them suitable for many different experimental scenarios. The evaluation of those forces, however, is still a bottleneck due to the numerical methods seen in the literature until now, which are usually slow and render low experimental yield. Here, we present BeadBuddy, a ready-to-use platform for the evaluation of deformation and stresses from fluorescently labeled sensors within seconds. The strengths of BeadBuddy lie in the precomputed analytical solutions of the elastic problem, the abstraction of data into spherical harmonics, and a simple user interface that creates a smooth workflow for force inference.
We believe in a more predictive and efficient drug development with reduced animal experiments!
This drives us every day to combine our various expertise in biology, physics, chemistry, and data science to provide innovative solutions for drug discovery!
Reliable, science-based, and extremely convenient for you!

Data Sciences

Biology

Biophysics

Chemistry

Biophysics
